Answers
;
Explanation: Look at the scrrn shot and you can see what is on there or not. :)))))
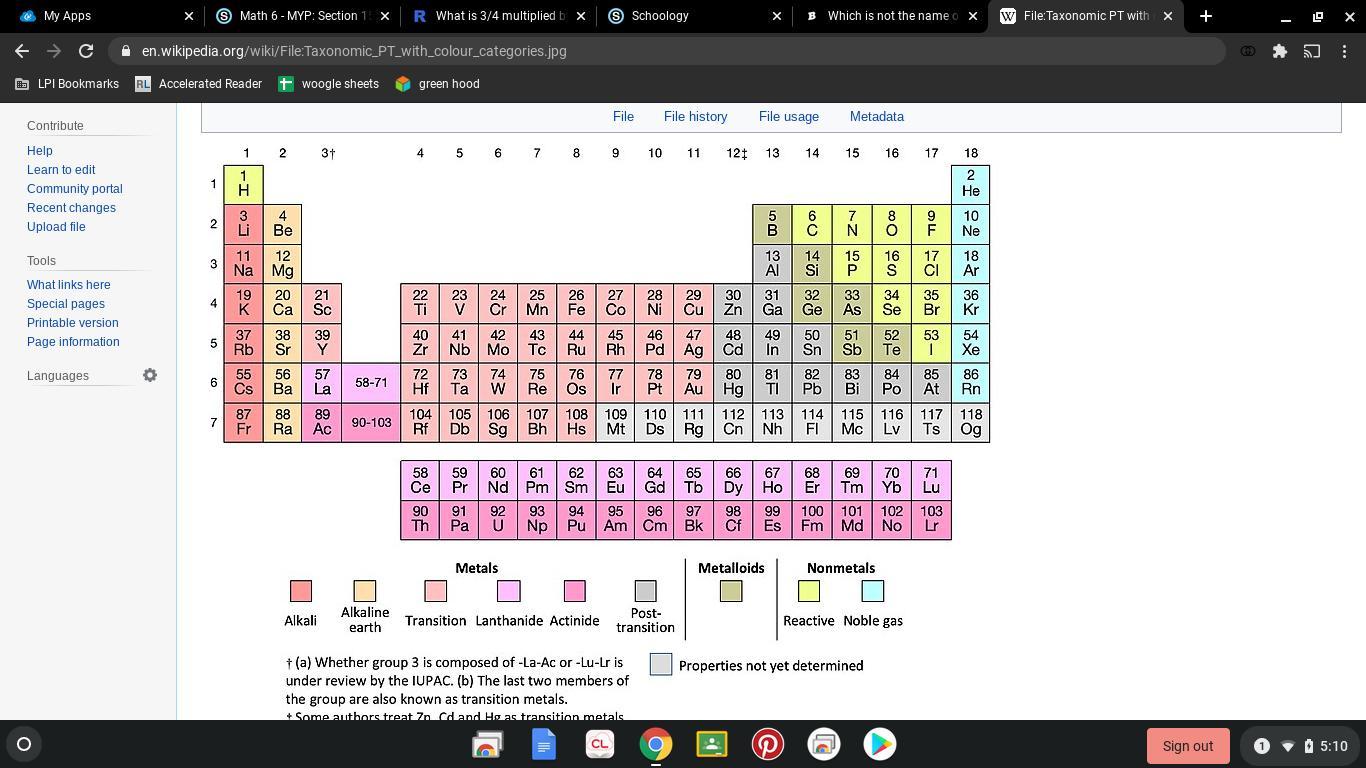
Actinides are not the name of the family of the periodic table.
Explanation:
In the modern periodic table :
Elements are arranged on the basis of their atomic numbersThere are 18 groups(vertical groups) and 7 periods (horizontal rows)Groups are also called families of the periodic table.Metals are present from the middle to the left-hand side of the periodic tableNonmetals are present on the upper right-hand side of the periodic tableMetalloids divide the periodic table in a zig-zag line with nonmetals on the right and metals on the left.The alkali metals are present under group 1The alkaline earth metals are present under group 2The noble gases are present under group 18The halogens are present under group 17The elements in separate two horizontal rows in the bottom are lanthanides and actinides.So, from this, we can conclude that actinides are not the name of the family of the periodic table.
Learn more about the periodic table:
brainly.com/question/226164?referrer=searchResults
brainly.com/question/1447213?referrer=searchResults
Related Questions
Calculate the following quantity: molarity of a solution prepared by diluting 49.16 mL of 0.0270 M ammonium sulfate to 525.00 mL.
Answers
Answer:
2.528x10⁻³M
Explanation:
Molarity is an unit of concentration used in chemistry. Is defined as the moles of solute per liter of solution.
To find the molarity of the solution we need to determine the moles of ammonium sulfate present in the initial 49.16mL solution and, with total volume, we can find the molarity, thus:
Moles ammonium sulfate:
49.16mL = 0.04916L * (0.0270 moles / L) =
1.327x10⁻³moles ammonium sulfate
These moles are present in 525.0mL = 0.525L. Thus, molarity of the solution will be:
1.327x10⁻³moles ammonium sulfate / 0.525L =
2.528x10⁻³MThe empirical formula of CBr2 has a molar mass of 515.46 g/mol. What is the molecular formula of this
compound
Answers
Answer:
C3Br6
Explanation:
C= (1 X 12.011) = 12.011
Br= (2 X 79.904)= 159.808
159.808+12.011 = 171.819
515.46 divided by 171.819 = 3.00
so you mulitpy CBr2 by 3 which gives you C3Br6
Which is the product of that reaction
Answers
Answer:
B
Explanation:
What is the frequency of a wave having 4.90 x 10 -12 J of energy?
Answers
Answer:
The answer is
[tex] \huge 7.40 \times {10}^{21} Hz[/tex]
Explanation:
To find the frequency of the wave we use the formula
[tex]f = \frac{E}{h} \\ [/tex]
where
E is the energy
f is the frequency
h is the Planck's constant which is
6.626 × 10-³⁴ Js
From the question
E = 4.90 × 10-¹² J
So we have
[tex]f = \frac{4.90 \times {10}^{ - 12} }{6.626 \times {10}^{ - 34} } \\ [/tex]
We have the final answer as
[tex]7.40 \times {10}^{21} \: \: Hz[/tex]
Hope this helps you
In which atmospheric layer is the ozone layer?
A.troposphere
B.mesosphere
C.stratosphere
D.thermosphere
Answers
Answer:
stratosphere
Explanation:
contains a high concentration of ozone in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere.
Answer:
stratosphere
Explanation: Most atmospheric ozone is concentrated in a layer in the stratosphere, about 9 to 18 miles (15 to 30 km) above the Earth's surface. Ozone is a molecule that contains three oxygen atoms.
Which is a chemical property of milk
A. Milk has a ph ranging from 6.4 to 6.8
B. Milk spoils when left unrefrigerated
C. Milk boils at about 212F
D. Milk curdles when mixed with vinegar
Answers
Answer:
C. Milk boils at about 212F
Explanation:
The principal constituents of milk are water, fat, proteins, lactose (milk sugar) and minerals (salts). Milk also contains trace amounts of other substances such as pigments, enzymes, vitamins, phospholipids (substances with fatlike properties), and gases.
C is the answer milk boils about 212F
A sample of propane, C3H8, contains 13.8 moles of carbon atoms. How many total moles of atoms does the sample contain
Answers
Answer:
[tex]Total = 50.6\ moles[/tex]
Explanation:
Given
[tex]Propane = C_3H_8[/tex]
Represent Carbon with C and Hydrogen with H
[tex]C = 13.8[/tex]
Required
Determine the total moles
First, we need to represent propane as a ratio
[tex]C_3H_8[/tex] implies
[tex]C:H = 3:8[/tex]
So, we're to first solve for H when [tex]C = 13.8[/tex]
Substitute 13.8 for C
[tex]13.8 : H = 3 : 8[/tex]
Convert to fraction
[tex]\frac{13.8}{H} = \frac{3}{8}[/tex]
Cross Multiply
[tex]3 * H = 13.8 * 8[/tex]
[tex]3 H = 110.4[/tex]
Solve for H
[tex]H = 110.4/3[/tex]
[tex]H = 36.8[/tex]
So, when
[tex]C = 13.8[/tex]
[tex]H = 36.8[/tex]
[tex]Total = C + H[/tex]
[tex]Total = 13.8 + 36.8[/tex]
[tex]Total = 50.6\ moles[/tex]
If 5.00g of iron metal is reacted with 0.950g of Cl2 gas, how many grams of ferric chloride (FeCl3) will form?
Answers
Answer:
1.45g of FeCl3
Explanation:
The equation of the reaction is given as;
2Fe + 3Cl2 --> 2FeCl3
2 mol of Fe reracts with 3 mol of Cl2 to form 2 mol of FeCl3
Upon converting to mass using;
Mass = Number of moles * Molar mass
( 2 * 55.85 = 111.7g ) of Fe reacts with ( 3 * 71 = 213g ) of Cl2 to form ( 2 * 162.2 = 324.4g) of FeCl3
Cl2 is the limiting reactant as it determines how much of FeCl3 is formed
213g of Cl2 = 324.4g of FeCl3
0.950g of Cl2 = x
x = (0.950 * 324.4 ) / 213
x = 1.45g of FeCl3
Discuss the relationship between atoms, elements and compounds. Include in your discussion if these are mixtures or pure substances and why.
Answers
Answer:
Elements are the simplest complete chemical substances. Each element corresponds to a single entry on the periodic table. An element is a material that consists of a single type of atom. Each atom type contains the same number of protons.
Chemical bonds link elements together to form more complex molecules called compounds. A compound consists of two or more types of elements held together by covalent or ionic bonds.
Explanation:
How do the test variables (independent variables) and outcome variables (dependent variables) in an experiment compare? A. The test variables (independent variables) and outcome variables (dependent variables) are the same things. B. The test variable (independent variable) controls the outcome variable (dependent variable). C. The test variable (independent variable) and outcome variable (dependent variable) have no affect on each other. D. The outcome variable (dependent variable) controls the test variable (independent variable).
Answers
Answer:
I'm on the exact same queston
Answer:
The test variable (independent variable) controls the outcome variable (dependent variable)
Explanation:
its right on study island
10. Which of the following is NOT correctly matched?
A. Salt: Na and C: Element
B. Water: H and O: Corapound
C. Carbon dioxside: C and O: Compound
D. Magnesium chloride: Mg and C: Compound
Answers
Answer:
A. because salt : Na and Cl, not C
Level 1 01 Which correctly pairs the outside particles with their charge? A. Electrons: Positive B. Protons: Positive C. Neutrons: Neutral D. Electrons: Negative
Answers
Answer:
D. Electrons: Negative.
Explanation:
Hello, happy to help you today!
In this case, by considering the Bohr's atomic model in which atom is composed by a nuclei containing both protons and neutrons which are positively and neutrally charged respectively and surrounding electrons assembled in orbits or levels of energy which are negatively charged in order to provide a balance to the atom, the correct statement is: D. Electrons: Negative. Also consider the Bohr's model on the attached picture.
My best regards to you!
which of the following Ph levels would indicate the weakest base?
15
8
6
2
Answers
Answer:
8
Explanation:
7 is neutral any anything above it is basic and anything below is acidic which means 8 would be the lowest base
Answer:
The pH of a weak base falls somewhere between 7 and 10.
Explanation:
Like weak acids, weak bases do not undergo complete dissociation; instead, their ionization is a two-way reaction with a definite equilibrium point
How to separate given mixture?
Answers
Answer:
Chromatography involves solvent separation on a solid medium.
Distillation takes advantage of differences in boiling points.
Evaporation removes a liquid from a solution to leave a solid material.
Filtration separates solids of different sizes.
Explanation:
One way to represent a substance is with a chemical formula. In the formula CO2, what do the symbols Cand o refer to?
Answers
Answer:
C is for carbon and O is for oxygen
6. Clownfish, sea anemone, coral, sea urchins, sponges and parrot fish are 5 points
just a few examples of the large variety of organisms that can be found in
a coral reef. The coral reef and its inhabitants (residents) are suffering due
to several factors such as pollution, coastal development, sedimentation
and destructive fishing practices. What would be the most likely result if
some of the species in the coral reef are destroyed? *
Answers
Answer:
The orange-and-white clownfish you know from Hollywood is only one of 30 species of clownfish that live in coral reefs around the world; small in size and big in personality, these tiny comedians have one of the most unique lifestyles in the reef. The orange-and-white clownfish you know from Hollywood is only one of 30 species of clownfish that live in coral reefs around the world; small in size and big in personality, these tiny comedians have one of the most unique lifestyles in the reef.
Explanation:
point being, it would be absolutely terrible
Coral reef are the biggest biodiversity in the world. Therefore, destruction of coral reefs definitely affect the aquatic livings. As well as it leads to erosion of seashore lines.
What are coral reefs?Corals reefs are the habitat of 25% of sea organisms. It means there are well over a million species that rely on coral reefs for their existence. The reef offers these organisms the vital food, habitat, and breeding grounds required for the survival of their species.
The marine biodiversity would suffer greatly if their homes vanished. And many fish, turtles, and other species would vanish in a domino-like fashion.
Coral reefs are threatened by a number of things, such as local threats like overfishing, destructive fishing methods, coastal development, pollution, and careless tourism, as well as global effects of climate change like warming seas and rising CO₂ levels in the water. Natural phenomena like hurricanes, El Nio, and diseases are also a threat to oral reefs.
Find more on coral reefs:
https://brainly.com/question/364711
#SPJ6
A tree frog uses plants or trees for protection from the rain. The frog is protected from the rain and the tree is neither helped nor harmed
Answers
A black object appears black because it reflects all light , and does not absorb any light. True or False
Answers
Answer:
False
Explanation:
It is the other way round, a black object appears black because it absorbs all the wavelength of light and does not reflect any light.
A black bucket will appear black because, it absorbs all wavelength of light incident on it. No other wavelength is reflected from the surface of the body. A black color suggests that a body is not reflecting any color. Instead such a body absorbing all the colors. For a white object, they reflect all the wavelength that is incident upon them.If 1.4434 moles of H2O are produced, how many moles of N2 will also be produced?
Answers
Answer:
1 gram of N2 is equal to 0.035697202053303 mole. i dont know the rest but intried to help
. hopefully this will give you someone else a starting point. goodluck
Define waves in your own words.
Answers
Answer:
Waves is the disturbance or variation that transfer energy from one location to other
Answer:
this is not in my words but i think this will help
(put some of the words you would use in this)
Explanation:
Transverse waves are always characterized by particle motion being perpendicular to wave motion. A longitudinal wave is a wave in which particles of the medium move in a direction parallel to the direction that the wave moves. ... Longitudinal waves are always characterized by particle motion being parallel to wave motion.
hope i helped
When 435 J of heat is added to 3.4 g of olive oil that's at 21 Deg C, it's
temperature increases to 85 Deg C. Calculate the specific heat of Olive oil? Show work
Answers
Answer:
k Nishant
Explanation:
i don't know sorry but u can search in google
What type of energy is defined as the kinetic energy of the atoms of a substance?
Answers
Explanation:Thermal energy, or heat, is the energy that comes from the movement of atoms and molecules in a substance. Heat increases when these particles move faster. Geothermal energy is the thermal energy in the earth. Motion energy is energy stored in the movement of objects.
I hope this helped!
Which of the following substances would have the greatest ductility?
A. Fe(s)
B. SiO2(s)
C. C(s)
D. NaCl(s)
Answers
Fe(s) would have the greatest ductility.
What is ductility?Ductility is the capability of a fabric to be drawn or plastically deformed without fracture. it's far therefore a demonstration of how 'gentle' or malleable the fabric is. The ductility of steel varies relying on the sorts and levels of alloying factors gift.
What are malleability and ductility?Ductility is the property of metallic associated with the capability to be stretched into twine without breaking. Malleability is the assets of metallic associated with the ability to be hammered into thin sheets without breaking. The outside force or strain is tensile pressure.
Learn more about ductility here https://brainly.com/question/496496
#SPJ2
Which option BEST explains how thermal equilibrium interacts with heat transfer between particles?
a
Thermal equilibrium stops the transfer of energy in just one direction when both objects reach the same temperature, but allows their particles to continue transferring that energy back and forth.
b
Thermal equilibrium always transfers energy from the hotter object to the colder one, and increases the energy and speed of moving particles in both objects as the temperature decreases.
c
Thermal equilibrium helps the transfer of energy between the particles of some materials better than others, but always stops the transfer of energy in materials like plastic and wood.
d
Thermal equilibrium quickly transfers energy back to the particles of the object that was originally hotter, and requires that the particles in both objects have reached equal energy and density.
Answers
Answer:
Thermal equilibrium stops the transfer of energy in just one direction when both objects reach the same temperature, but allows their particles to continue transferring that energy back and forth.
Explanation:
a. The transfer of energy occurs until both objects reach thermal equilibrium. But particles are always moving and crashing with each other. TRUE.
b. The heat transfer occurs from the hotter object to the colder one but moving of particles descreases with temperature decreasing. FALSE.
c. Plastic and wood have a poor quality to transfer energy but there is no material that "stops" perfectly the transfer of energy. FALSE.
d. The heat is transferred from the particles of the hotter object to the particles of the colder one. FALSE
If 25.6 mL isopropyl alcohol fully decomposes, what mass of H2 is formed? The density of isopropyl alcohol is 0.785 g/mL. g
Answers
Answer:
The correct answer is 0.67 g H₂
Explanation:
Isopropyl alcohol (C₃H₇OH) can decompose to give acetone (C₂H₆OH) and hydrogen gas (H₂) according to the following chemical equation:
C₃H₇OH (g) ⇒ C₂H₆CO(g) + H₂(g)
We can calculate the initial mass of isopropyl alcohol from the density and volume data:
density = m/V = 0.785 g/mL
⇒ m = density x V = 0.785 g/mL x 25.6 mL = 20.096 g C₃H₇OH
According to the chemical equation 1 mol of C₃H₇OH gives 1 mol H₂. The molar mass of C₃H₇OH is:
molar mass C₃H₇OH = (12 g/mol x 3) + (1 g/mol x 7) + 16 g/mol + 1 g/mol = 60 g/mol
molar mass H₂ = 1 g/mol x 2 = 2 g/mol
So, we obtain: 2 g H₂ from 60 g C₃H₇OH. We multiply this stoichiometric ratio (2 g H₂/60 g C₃H₇OH) by the initial mass of C₃H₇OH to obtain the mass of H₂ is formed:
20.096 g C₃H₇OH x (2 g H₂/60 g C₃H₇OH) = 0.6698 g ≅ 0.67 g H₂
how can you tell where sugar enters the blood stream
please help asap
Answers
Answer:
Sugar can't enter cells directly , so when blood sugar level rises, ... signal for the release of insulin into the bloodstream.
Explanation:
yes when the sugar enter the bloodstream it may slowly slowly effect in your body and it may causes diabetes
Which is an example of a current research focus in chemistry?
A. applying gene therapy to treat certain diseases
B. using hook-and-loop tape in the clothing industry
C. developing smoke detectors for common use
D. studying coal combustion as an energy source
Answers
Answer:
b is the correct answer
do not trust answer one
Explanation:
3 points
18) A student determines the density of gold to be 20.9g/L. The true
density of gold is 19.30g/L. What is the student's percent error?round
answer to 2 significant figures *
Answers
Answer:
The answer is 8.29 %Explanation:
The percentage error of a certain measurement can be found by using the formula
[tex]P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ [/tex]
From the question
actual density = 19.30g/L
error = 20.9 - 19.3 = 1.6
We have
[tex]p(\%) = \frac{1.6}{19.3} \times 100 \\ = 8.290155440...[/tex]
We have the final answer as
8.29 %Hope this helps you
for the following reaction, provide the missing information
Answers
Answer:
19. Option B. ⁰₋₁B
20. Option D. ²¹⁰₈₄Po
Explanation:
19. ²²⁸₈₈Ra —> ²²⁸₈₉Ac + ʸₓZ
Thus, we can determine ʸₓZ as follow:
228 = 228 + y
Collect like terms
228 – 228 = y
y = 0
88 = 89 + x
Collect like terms
88 – 89 = x
x = –1
Thus,
ʸ ₓZ => ⁰₋₁Z => ⁰₋₁B
²²⁸₈₈Ra —> ²²⁸₈₉Ac + ʸₓZ
²²⁸₈₈Ra —> ²²⁸₈₉Ac + ⁰₋₁B
20. ᵘᵥX —> ²⁰⁶₈₂Pb + ⁴₂He
Thus, we can determine ᵘᵥX as follow:
u = 206 + 4
u = 210
v = 82 + 2
v = 84
Thus,
ᵘᵥX => ²¹⁰₈₄X => ²¹⁰₈₄Po
ᵘᵥX —> ²⁰⁶₈₂Pb + ⁴₂He
²¹⁰₈₄Po —> ²⁰⁶₈₂Pb + ⁴₂He
On average, about ____________ of incoming solar radiation is reflected back to space.
A 50%
B 30%
C 20%
D 10%